Oxford BioDynamics
EpiSwitch PSE
EpiSwitch PSE
EpiSwitch® Prostate Screening Test (EpiSwitch PSE) - The 94% accurate prostate cancer blood test.
Testing with a standard PSA test is 55% accurate. The PSE test boosts the prostate cancer screening test to 94% accuracy.
1 in 8 men will get prostate cancer in their lifetime. If you have received an elevated PSA result, or have concerns where prostate cancer must be ruled out, talk to your primary care doctor about the PSE test and www.94percent.com
BENEFITS
BENEFITS
DOWNLOADS
DOWNLOADS
HOW TO ORDER
HOW TO ORDER


EpiSwitch Prostate Screening Test (PSE)
The 94% accurate prostate cancer blood test
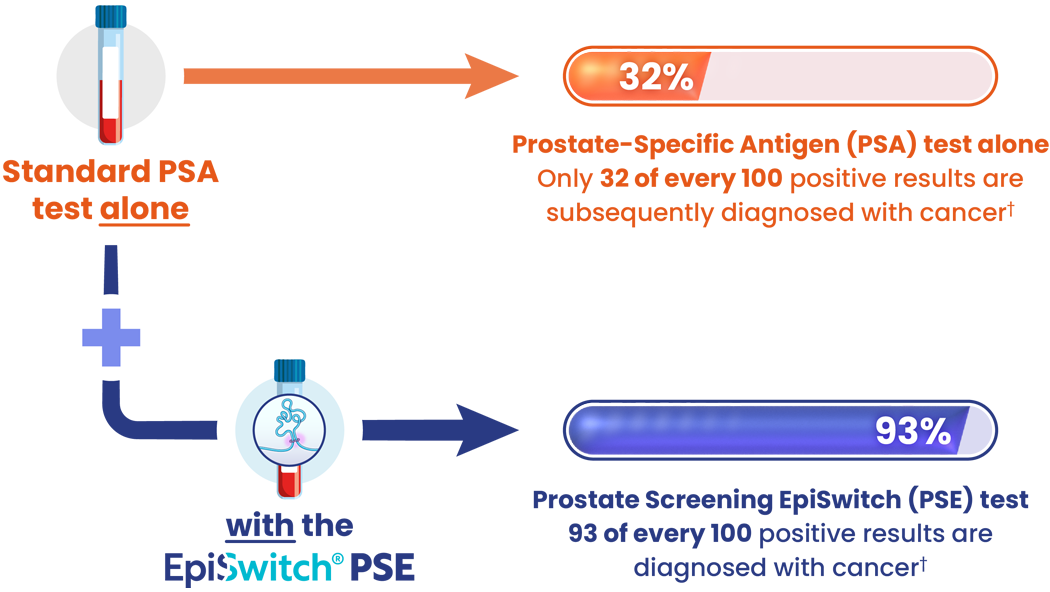
PSE can reduce referrals for unnecessary prostate biopsies
Source: † Pchejetski et al. (2023). Circulating CCS Significantly Enhance PSA PPV and Overall Accuracy for Prostate Cancer Detection. Cancers, 15(3), 821. http://dx.doi.org/10.3390/cancers15030821
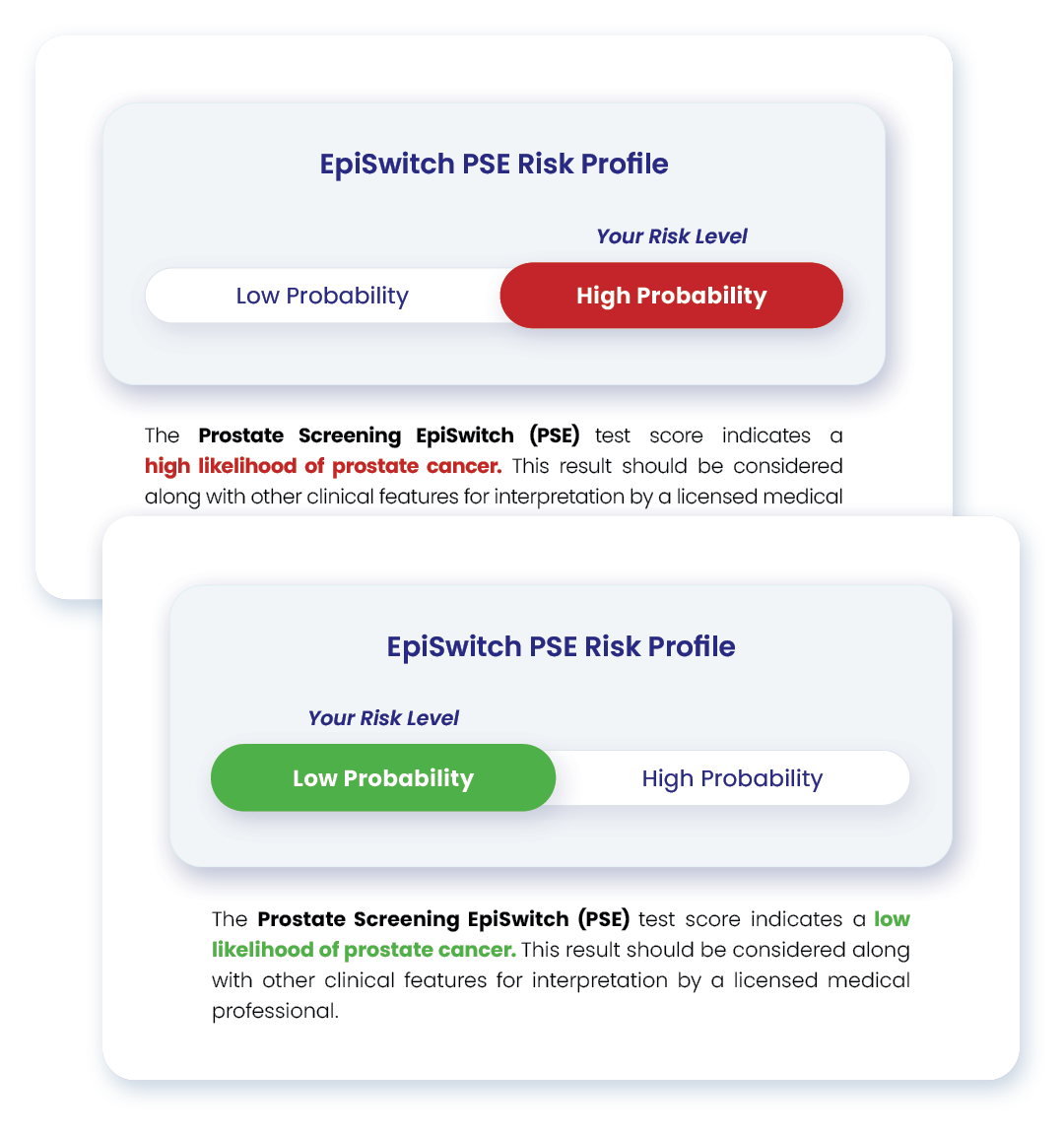
Personalised EpiSwitch PSE report
Personalize your guidance for each patient
An EpiSwitch PSE report is securely returned to the ordering physician. It includes indications for the healthcare professional to determine who should proceed to biopsy, and who can be placed on active surveillance.
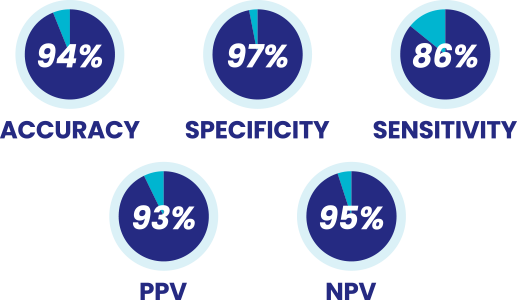
Validated test with high accuracy, specificity and sensitivity
- EpiSwitch PSE is administered alongside or following a standard PSA test
- Blood test measures five (5) epigenetic biomarkers and combines these with the patient’s PSA score
- EpiSwitch PSE was validated across a population of asymptomatic men randomly invited for cancer screening
Source: † Pchejetski et al. (2023). Circulating CCS Significantly Enhance PSA PPV and Overall Accuracy for Prostate Cancer Detection. Cancers, 15(3), 821. http://dx.doi.org/10.3390/cancers15030821

Fast and accurate prediction of an individual's likelihood of prostate cancer with a routine qPCR blood test.
Our comprehensive and validated approach delivers a report to the ordering physician via secure electronic communication or fax. Results will be available around 5 days after the lab receives the patient's blood sample.
Built on Oxford BioDynamics' EpiSwitch technology platform, PSE is based on a comprehensive and validated approach
The EpiSwitch PSE blood signature was developed using Oxford BioDynamics' EpiSwitch 3D genomic immune health database—the world's largest genomic database—via several controlled studies.
PSE uses 3D genomic profiling to assess an individual's current likelihood of prostate cancer. This method uncovers vital drivers of systemic changes in their blood, including immune cells associated with prostate cancer.
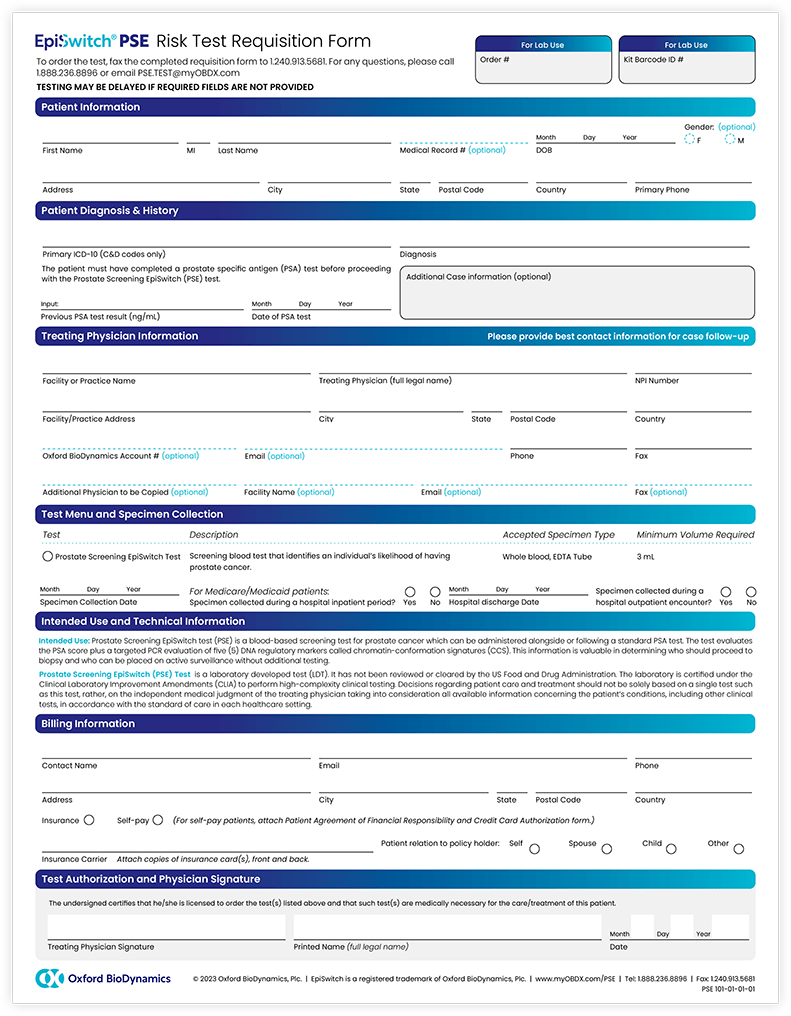
Order the EpiSwitch PSE test
Are you interested in becoming an EpiSwitch PSE registered healthcare provider?
Contact EpiSwitch PSE Customer Service at 888-236-8896 (US) or 01865 504932 (UK) to open an account, request customized EpiSwitch PSE Requisition Forms branded for your practice, order EpiSwitch PSE collection kits, or request further assistance relating to EpiSwitch PSE. We look forward to speaking with you.
Frequently Asked Questions about EpiSwitch PSE
How was EpiSwitch PSE developed?
The EpiSwitch PSE was developed using the EpiSwitch 3D genomics platform and validated across multiple studies, including a screening study population run with Imperial College Healthcare NHS Trust comprised of asymptomatic men randomly invited for cancer screening. Learn more here.
How do I order EpiSwitch PSE?
Contact Oxford BioDynamics at 888-236-8896 (US) or 01865 504932 (UK) to setup an account today. Alternatively, send in a completed Requisition Form (US) or Requisition Form (UK) via fax to 240-913-5681, and we will contact you promptly.
How is EpiSwitch PSE different from other cancer diagnostic tests?
The EpiSwitch PSE is a blood test designed to significantly enhance the accuracy of prostate cancer detection when administered alongside or after a standard PSA test; it doesn't require a prostate biopsy.
This test utilizes 3D genomic profiling to identify an individual's current likelihood of prostate cancer by uncovering crucial drivers of personal systemic changes in their blood, including immune cells associated with prostate cancer.
When used alongside and following a standard PSA test, the EpiSwitch PSE generates results that can be trusted to accurately determine the likelihood of prostate cancer and reduce the number of men referred for unnecessary, invasive, and expensive diagnostic procedures.
Where is EpiSwitch PSE performed?
EpiSwitch PSE is performed in a CLIA-certified laboratory. Test results are securely reported to the ordering physician in 5 days or less after the lab receives the patient's blood sample.
Which individuals benefit most from EpiSwitch PSE?
This test is beneficial for men undergoing prostate cancer screening with their physician. The EpiSwitch PSE is a routine blood test that assesses an individual's current likelihood of having prostate cancer.
When combined with a standard PSA test, the EpiSwitch PSE produces reliable results to accurately determine the probability of prostate cancer. This valuable information enables doctors to identify who should undergo a biopsy and who can be placed on active surveillance without the need for further testing.
How will I receive the results of EpiSwitch PSE?
EpiSwitch PSE results are provided as a report that is delivered to the ordering physician via secure electronic communication or fax. Results will be available around 5 days after the lab receives the patient's blood sample.
Can the results of EpiSwitch PSE be sent directly to patients?
No. To ensure doctor-patient confidentiality and maintain rigorous patient-data privacy standards, we securely provide EpiSwitch PSE results directly to the ordering physician only.
What is 3D genomics?
It is a type of genomic testing that looks at the regulation of an individual’s genes at the level of changes in its three-dimensional (3D) conformation. Information about an individual’s genomic conformation tells us the likelihood of prostate cancer being present or absent. Learn more about 3D genomics here, and 3D genomic regulatory changes in prostate cancer here.

